Konstantinos Poulakis, Joana B. Pereira, J.-Sebastian Muehlboeck, Lars-Olof Wahlund, Örjan Smedby, Giovanni Volpe, Colin L. Masters, David Ames, Yoshiki Niimi, Takeshi Iwatsubo, Daniel Ferreira, Eric Westman, Japanese Alzheimer’s Disease Neuroimaging Initiative & Australian Imaging, Biomarkers and Lifestyle study
Nature Communications 13, 4566 (2022)
doi: 10.1038/s41467-022-32202-6
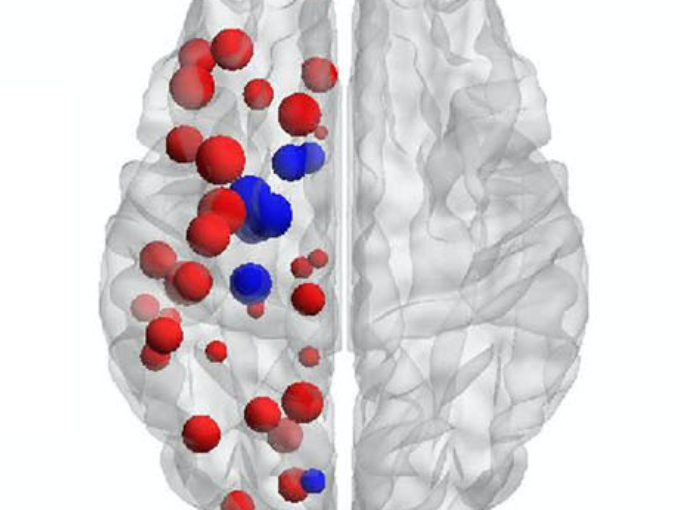
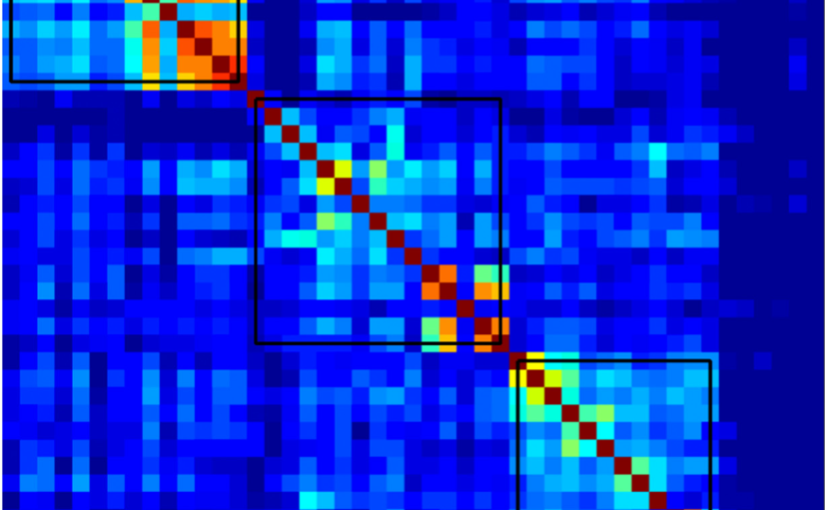
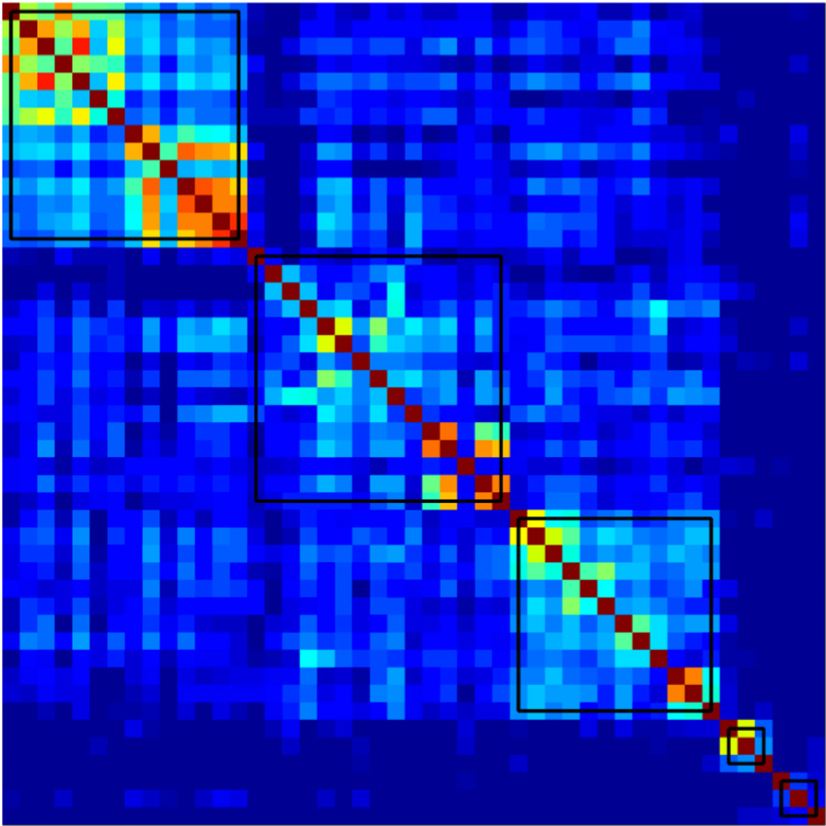
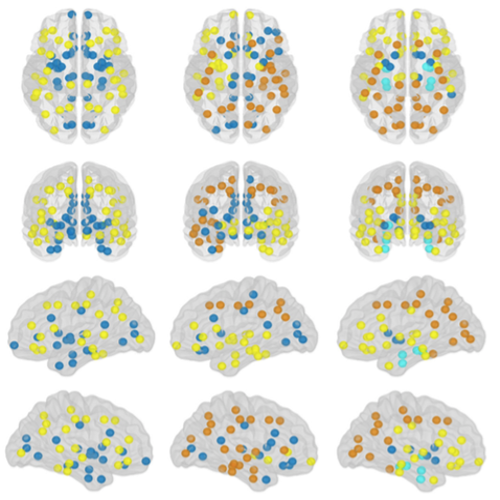
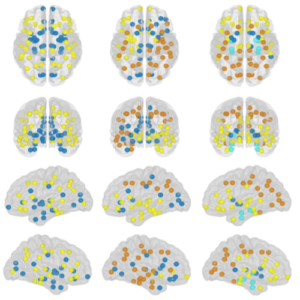
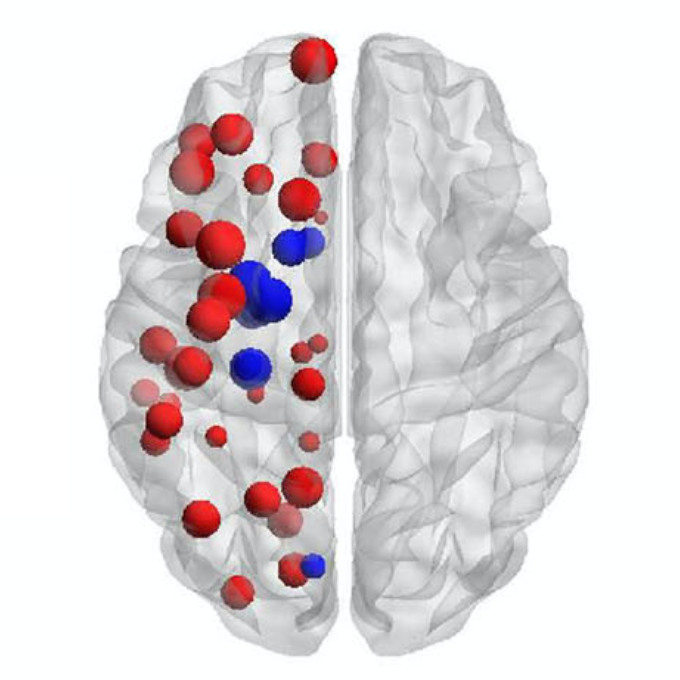
Understanding Alzheimer’s disease (AD) heterogeneity is important for understanding the underlying pathophysiological mechanisms of AD. However, AD atrophy subtypes may reflect different disease stages or biologically distinct subtypes. Here we use longitudinal magnetic resonance imaging data (891 participants with AD dementia, 305 healthy control participants) from four international cohorts, and longitudinal clustering to estimate differential atrophy trajectories from the age of clinical disease onset. Our findings (in amyloid-β positive AD patients) show five distinct longitudinal patterns of atrophy with different demographical and cognitive characteristics. Some previously reported atrophy subtypes may reflect disease stages rather than distinct subtypes. The heterogeneity in atrophy rates and cognitive decline within the five longitudinal atrophy patterns, potentially expresses a complex combination of protective/risk factors and concomitant non-AD pathologies. By alternating between the cross-sectional and longitudinal understanding of AD subtypes these analyses may allow better understanding of disease heterogeneity.